Calculation of the properties
If air and water are present together in a confined space, the condition of equilibrium is reached where the air becomes saturated water steam. If the temperature of the mixture is known, the pressure of water vapor will vapour pressure at this temperature (Table. 20.1). Dalton's law of partial pressures (see also Section 1.6) States that the total pressure of a mixture of gases equals the sum of the pressures of individual constituent gases, adopted at the same temperature and takes the same amount. Since water vapour pressure depends on temperature, this pressure can be obtained from the steam table, as shown below. The partial pressure of dry air, therefore, needs to be balance.
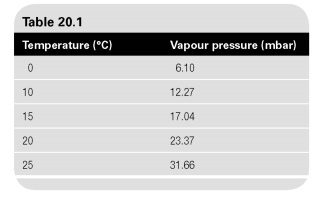
Thus, for the air-water mixture of vapors at 25C:
- General (standard) pressure = 1013.25 mbar
- The partial pressure of saturated vapors = 31.66 mbar
- The partial pressure of dry air = 971.59 mbar
Specific enthalpy (or total heat) the mixture can be taken from 0 K (-273.15C) or from any convenient arbitrary zero.
Since most air conditioning processes occur above the freezing point of water, and we are concerned mainly the differences, rather than absolute values, reference, as a rule, is carried out 0C, dry air. Under the terms of the 25C, rich, specific enthalpy of mixture per kilogram of dry air,
- Sensible heat of dry air = 1.006 X 25 = 25.15 kJ/kg
- Sensible heat of water = 0.020 16 X 25 X 4.187 = 2.11
- Latent heat of water = 0.02016 X 2440 = 49.19
- Total 76.45 kJ/kg
..
|

