Boiling point
The temperature at which a liquid boils not constant, but varies depending on pressure. Thus, while the temperature of boiling water is assumed to be 100C, this is only true at a pressure of one standard atmosphere (1.013 bar) and the change of pressure, evaporating temperature, can be changed (table 1.1). This pressure-temperature property can be represented graphically (see Fig. 1.4).
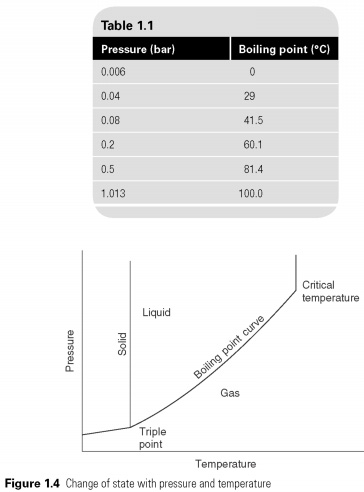
The boiling point of a substance is limited to the critical temperature at the upper end, for which it cannot exist in the liquid state and the triple point in the lower part, which freezing the temperature. Between these two limits, if a liquid at a pressure above the pressure boiling, it will remain in a liquid, so it will be supercooled below saturation, and if the temperature above the saturation, it will be gas and superheated. If both liquid and vapor on vacation in one case, and no other volatile substances, the condition must lie on a line of saturation.
Pressure below the triple point of pressure, solid body can directly modify gas (sublimation) and gas can change directly to the firm, both in the formation РґРІСѓРѕРєРёСЃСЊ carbon snow from the released gas.
Liquid zone to the left of the boiling point of the line to the supercooled liquid.
In the refrigeration term " saturation " is used to describe the liquid/vapor border, saturated vapour be presented in the form of lines and superheated the pair below the line.
....
|

