The Principle Of Continuous Absorption System
Absorption system uses ammonia, water and hydrogen. When he cooling provides constant, this is called continuous absorption system. In the continuous absorption system, ammonia refrigerant and solution of ammonia and water absorbent. A weak solution contains as much ammonia as possible. (A weak solution weak in its ability to absorb.) Strong solution contains less ammonia than a weak solution and strongly absorb more of ammonia. Continuous cooling cycle operates in automatic mode through the use of automatic control.
Many companies have variants of the base system. However, the principle remains the same. Torch lit, and the heat applied to the generator (figure 1 Fig. 17-4). Ammonia vapor is then released from the solution. This hot steam passes up through the percolator tube 2. This decision is carried away on the upper level separator 3.
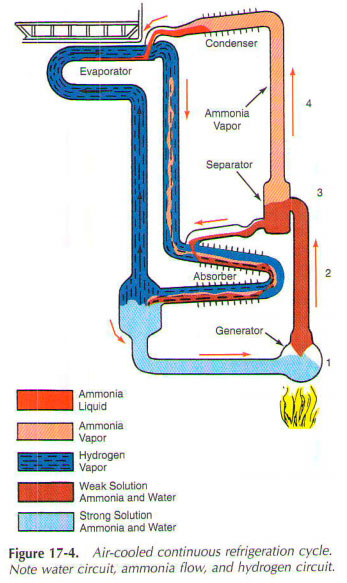
Most of the liquid solution is deposited at the bottom of the separator and into the sink.
Hot ammonia vapor light. It rises to the top of the tube, marked 4, in the condenser. Hot ammonia vapor then condenses to a liquid. Ammonia is now in a clean condition and it flows into the evaporator.
Ammonia is through liquid ammonia pipe and enters the evaporator. (This is because the fluid will always seeks its own level.) In the evaporator, it forms a large, shallow pools series horizontal guides. Large amounts of hydrogen gas, supplied to evaporator allow ammonia evaporates. This evaporation occurs at low pressure and low temperature (Dalton principle). During this process, the evaporation of ammonia absorbs heat from the food compartment the refrigerator. This causes the water in the ice cube containers for freezing. More hydrogen and less ammonia, the lower the temperature. Evaporating ammonia mixture with hydrogen.
Meanwhile, the weak solution of ammonia and water by gravity from the separator, paragraph 3. It flows down at the top of the absorber. At the top of the absorber, the solution corresponds to a mixture of hydrogen and ammonia vapor coming from the evaporator. Weak and a pretty cool solution absorbs ammonia vapors. Hydrogen is still free, because it will not mix with water. Because hydrogen is also very light, he now rises to the top of the absorber. From there he returned to the evaporator.
Absorber has a cooling fins air. Cooling weak solution helps absorb ammonia gas from ammonia, steam-hydrogen in the gas mixture. As absorbs water ammonia vapors released considerable amount of heat. Air cooled delete fins, this heat to allow refrigeration to continue. Liquid ammonia and water mixture is then fed back into the generator. There, the cycle begins again.
This device represents the welded construction. No moving parts to wear out or exit the settings. Total pressure throughout the entire cycle is about 400 psi (415 psia or 2864 kPa). This service (room) temperature of 100F (38C). So the design should be strong, to ensure a long life.
Carried out at temperatures 0F (18C) in the evaporator, ammonia should boil 15.7 psig inch (30.4 psia of $ 209.8 or kPa). This means hydrogen must pay the rest amount of pressure. (It would be 384,3 psig [2651.7 kPa], if the total pressure of 400 pounds per square inch.) This refrigerator is considered unique among household refrigerators...
|

