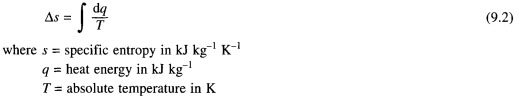Thermodynamics and cooling
The subject of thermodynamics was considered in part, in Chapter 2, it belongs to the physics of air-water vapor mixture. It is necessary to reconsider the topic here, as it relates to the behavior of refrigerant vapors in compression refrigeration cycles, in order to address such cycles quantitatively. As a preamble to this, some principles and definitions should be reviewed.
(a) Thermodynamics. This is simply the study of change relating to energy, but it is also determined by ASHRAE (1997), and the study of energy and its transformations, and its relation to the States of matter.
(b) a Thermodynamic system. This is determined by ASHRAE (1997) as " ... a region of space and / or quantity of matter, bounded closed surface'. There are two types of systems, which will be considered: closed systems, where there is no exchange of substances with the environment, and open systems, where a junction. With a closed system, the mass within the boundaries of the system remains constant, as in hermetic refrigerating installations.
With an open system, there is a mass flow through the boundary of the system, for example with a pumping process, which is the fluid in the system from the environment.
(c) the first law of thermodynamics. Otherwise interpreted as conservation of energy, this law States that energy can neither be created nor destroyed.
To open system, under steady state conditions, the units of measurement of mass flow rate of a pure substance, the first law is expressed steady stream of energy equation:

Please note that equation (2.19) offers an alternative way of expressing enthalpy.
(d) the second law of thermodynamics. In simple terms, this means that only the flows of heat from a higher temperature to a lower temperature. More formally, Spaulding and Cole (1961) States: it is impossible for a system that runs in a loop, to have as a sole effect, the transfer of heat from a low temperature system temperature.
(e) Heat. Energy was described ASHRAE (1997) as ability to produce the effect, and it can be saved, or in a transitional form. Potential energy is exemplified by such concepts as potential energy and kinetic energy, whereas heat is a form of energy transition. Heat can be defined as the interaction of two systems of different temperature and airflow is always from the higher to the lower temperature.
(f) Work. This aspect of energy. The work consists in the use of force by distance, energy transfer through the border between the two systems.
(g) the Entropy. It is a concept that is important when analyzing the behavior of thermodynamic systems. It is expressed in terms of the change in entropy is defined as the amount of heat crossing reversible systems, divided by the absolute temperature of the system, and gives
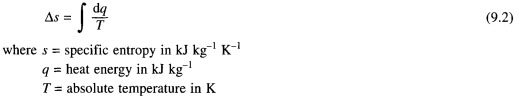
Entropy is the property of the system, it depends on the state of the substance. For a pure substance, its value can be set, as it is dependent on two independent properties, heat and the absolute temperature. Since it is defined as the difference, in equation (9.2), arbitrary zero must be adopted if she wants to be tabulated. It is, as a rule, at the temperature of zero degrees absolute.
Entropy can also be considered in terms of disorders of molecules in the system: if they disordered entropy more than if they in some sort of order. In addition, it can be viewed as having a certain amount of heat: in equation (9.2) shows that, if the entropy change of the small absolute temperature should be large, given the changes of heat. Therefore, you can build the absolute temperature, entropy diagram (see Fig. 9.4 and 9.5), in the issues that are of heat.
A process that occurs at constant entropy is called isentropic.
(h) Reversibility. Reversible process, which, upon completion, the back of the system and its surroundings to their original state. This does not apply to an irreversible process, an example of which is any process involving friction.
If there is no heat transfer with the surroundings and not internal friction losses, piston machine can act as a compressor or as an extension of the engine. Acting as a compressor power supply machine will be used for compression of gas processed. Acting as an extension car pressure difference between the inlet and outlet gas will be processed expand, driving a car. Machine for the liberation of the surroundings of the same power that was taken from them when the car acted as a compressor. Compression and expansion processes will then be reversible.
You can prove that the effectiveness of reversing the engine is always greater than the irreversible engine effect between two identical thermal collectors. Therefore it is desirable to refrigeration compressors should be performed convertible compression as possible...
|